WHO Tedros issues a medical product alert after Child Death toll surges to 66 in the Gambia after taking cough Syrups from Maiden Pharmaceuticals Ltd.
WHO Director-General Tedros Adhanom speaks on October 5, issuing a medical product alert on asking regulators to remove Maiden Pharma goods from the market.


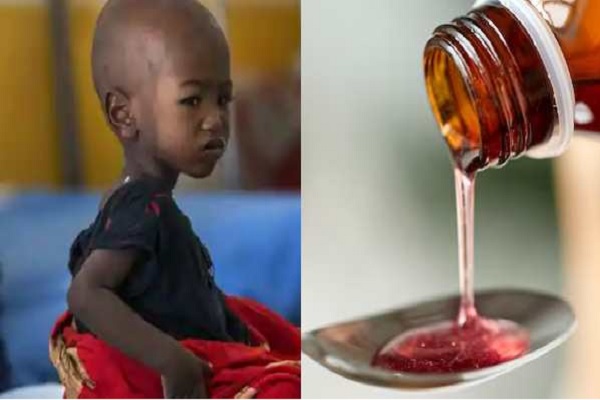
“The deaths of dozens of young children in the Gambia from acute kidney injuries may be linked to contaminated cough and cold syrups made by an Indian drug manufacturer”. World Health Organization said on Wednesday.
The findings, announced by WHO Director-General Tedros Adhanom Ghebreyesus, followed tests on several medicinal syrups that were suspected of causing 66 child deaths in the tiny West African country.
Tedros told reporters that the UN agency was investigating with Indian regulators and the company that made the syrups, New Delhi-based Maiden Pharmaceuticals Ltd.
Maiden Pharma declined to comment, while calls and messages to the Drugs Controller General of India went unanswered.
The WHO issued a medical product alert on Wednesday asking regulators to remove Maiden Pharma goods from the market.
The products may have been distributed elsewhere through informal markets, but had so far been identified only in the Gambia, the WHO said in its alert.
The alert covers four products: Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup.
Lab analysis confirmed unacceptable amounts of diethylene glycol and ethylene glycol, which can be toxic and lead to acute kidney injury, the WHO said.
Medical officers in the Gambia raised the alarm in July after dozens of children began falling ill with kidney problems.
The deaths confounded medics before a pattern emerged: dozens of patients younger than five were falling ill three to five days after taking a locally sold paracetamol syrup.
Gambia’s director of health services, Mustapha Bittaye, said similar problems have been detected in other syrups but that the ministry is awaiting confirmation of the results.
“The number of deaths has tapered off in recent weeks and the sale of these products made by Maiden Pharmaceuticals banned. However, until recently, some of the syrups were still being sold in private clinics and hospitals”. Mustapha reported.


Gambia’s Medicines Control Agency sent a letter on Tuesday to health professionals ordering them to stop selling any of the products listed by WHO.
Subscribe to our You Tube channel at Switch TV.
Maiden Pharmaceuticals manufactures medicines at its facilities in India, which it then sells domestically as well as exporting them to countries in Asia, Africa, and Latin America, according to its website.